Imagine a medicine that could be upgraded as effortlessly as installing an app on a smartphone. That is the revolutionary promise of “programmable drugs.” Unlike traditional therapies that act passively through chemical bonds, this new paradigm—driven by the twin engines of synthetic biology and artificial intelligence—translates DNA or RNA fragments into “genetic software.” Delivered by intelligent nanoscale carriers, these instructions can enter cells with pinpoint accuracy, rewriting and optimizing life’s operating code.
CRISPR as the “Scissors” and Nanoparticles as the “Rockets”:In the microscopic world, the CRISPR-Cas9 system functions like a precision pair of gene-editing scissors. When mounted on lipid nanoparticles—tiny “nano-rockets”—it can deliver customized DNA templates directly into the nucleus of diseased cells. Once an AI prediction model detects the molecular signals of disease, pre-programmed mRNA sequences launch a transcription-to-translation relay. Ribosomes, acting like miniature factories, then mass-produce therapeutic proteins—comparable to fleets of microscopic robots that can dismantle misfolded, disease-causing proteins and reawaken dormant tumor-suppressor genes.
Toward Cells with “Programmable Chips”:As research pushes forward, programmable drugs may one day act like installing a chip inside a cell—allowing diseased cells to be “rebooted” back into healthy ones. Scientists are now designing an integrated control system that spans gene editing, epigenetic tuning, and protein synthesis. In the future, this could resemble debugging computer code: restoring memory circuits in Alzheimer’s patients, reactivating motor neurons in those with ALS, and ultimately ushering in an era of personalized, digitally driven medicine.
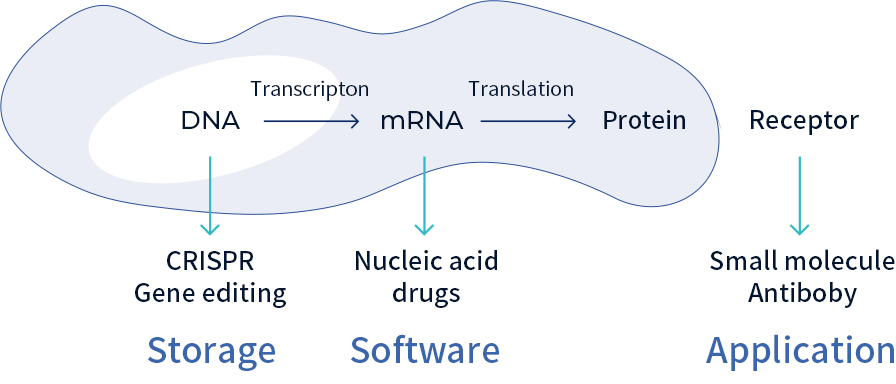
mRNA, short for messenger RNA, carries genetic instructions from DNA to the cell’s protein-making machinery. It acts as a bridge between genetic code and the proteins that sustain life. In theory, scientists can program mRNA to produce almost any protein, opening the door to treatments for a wide range of diseases.
In the theater of life, mRNA acts as a “dynamic score,” translating DNA’s static code into the living symphony of proteins. With AI-guided programmable drugs, scientists can rewrite this score, turning mRNA into a “molecular architect” that builds functional components inside cells on demand.
Scientists chemically modify mRNA to cloak it in an “invisibility cape,” helping it evade the immune system. They then load it into lipid nanoparticle “nano rockets” that deliver it directly to diseased tissues. Once there, the nanosystem senses environmental cues—such as the low oxygen levels in tumors—and releases the mRNA payload, instructing cells to produce therapeutic proteins.
Clinical trials have already demonstrated remarkable potential. For instance, mRNA encoding the Cas9 protein, together with guide RNA, forms a “gene-editing kit” capable of precisely removing faulty genes and replacing them with healthy ones. In therapies for rare diseases, mRNA can even reactivate fetal hemoglobin production, bypassing defective adult hemoglobin. Looking ahead, when combined with AI-driven predictions, mRNA therapies could dynamically adjust dosages in real time—much like a smart thermostat—enabling truly on-demand treatment.
The vision of “Programmable Cells”—a world free from disease and aging—depends on breakthroughs in nanomedicine delivery.
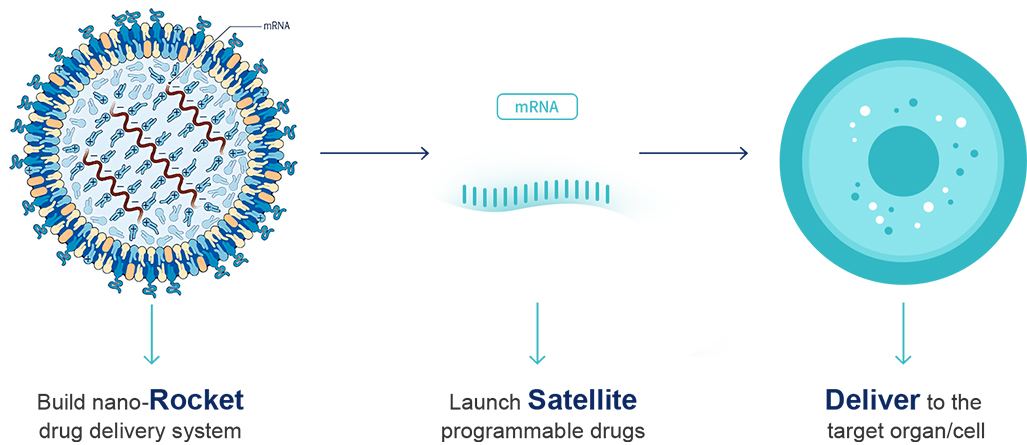
mRNA is a fragile “cotton satellite”: easily shredded by nucleases and too negatively charged to cross cell membranes. It needs the precise escort of an LNP nano-rocket. LNPs provide a three-layer defense: an outer PEG-lipid layer that disguises it as harmless lipoproteins to evade the immune system; a middle layer of ionizable lipids that fold tightly in acidic environments to protect the payload, then expand inside the cell’s basic environment to release it; and a structural core that grips and stabilizes the mRNA like molecular claws until delivery.
Future medicines will use genetic code as the central instruction set, delivered via nucleic acid delivery systems to precisely reprogram diseased cells. This “rocket + satellite” approach relies on specialized LNP nano-carriers—the rockets—loaded with nucleic acid payloads such as mRNA or CRISPR-Cas9 systems—the satellites—for targeted delivery and controlled release.
Lipid nanoparticles (LNPs) have revolutionized the way mRNA therapies are delivered. By dramatically improving both effectiveness and safety, LNPs were a key factor behind the success of Pfizer/BioNTech and Moderna’s COVID-19 vaccines, helping control the global pandemic. In 2022, pioneers of LNP drug delivery—Pieter Cullis, Katalin Karikó, and Drew Weissman—were honored with the prestigious Gairdner Award, often dubbed the “Little Nobel Prize.”
Designing effective LNPs is no small feat. Each particle typically contains at least four distinct lipid components, each with unique chemical and physicochemical properties. When these variables are combined, the resulting design space becomes vast and complex—finding the optimal combination can feel like searching for a needle in a haystack.
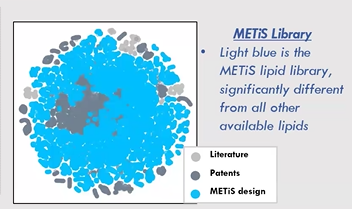
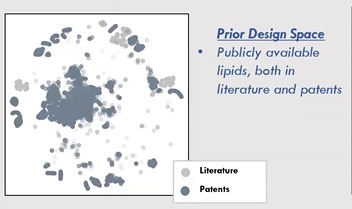
In reality, the most effective regions of this design space may be discontinuous and scattered. Traditional Design of Experiments (DOE) methods can explore only a limited area, often identifying local optima while missing the global best solutions. Historical data from literature and patents shows clusters of lipid designs, as researchers build on one another’s work. Yet large unexplored regions remain, offering untapped potential for innovation.
From day one, METiS TechBio set out to take a boldly different approach. By combining AI and machine learning with high-throughput experimental screening, the company sought to explore these uncharted territories—what they call the “blue ocean” beyond traditional methods.
Over the past five years, METiS has pioneered an end-to-end AI-driven lipid engineering platform. Compared to conventional approaches, their AI systems have expanded the nanomaterials design space by more than 1,000-fold, opening up entirely new possibilities for precision drug delivery.